Metallocene - Catalysts
Metallocenes
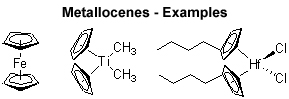
The metallocene icon of organometallic chemistry originally refers to bis(cyclopentadienyl)metal complexes (‘sandwich’), but wider usage is now accepted to include cyclopentadienyl complexes (‘half sandwich’) and multicyclopentadienyl complexes (‘multidecker sandwich’) as well as complexes with additional substitution at the metal center. [...] In fact, metallocene-like complexes are now known for many elements in the periodic table.
(Metallocenes Synthesis - Reactivity - Applications, A. Togni, R.L. Halterman(eds.))
Catalysts
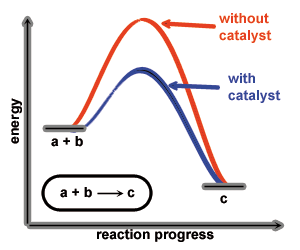
A catalyst increases the rate of a chemical reaction by lowering the activation energy of the reaction. Thereby the catalyst is not consumed or transformed by the reaction. Furthermore its participation can increase the selectivity of the chemical reaction it catalyses (ideally).
Catalysts are widely used in industrial processes which would not be realisable without catalysts. Also nature works with catalysts (enzymes) to increase the rate of biochemical processes and selectivity. Seen from an environmental point of view catalysts are one of the most important additives in chemical industry/processes.